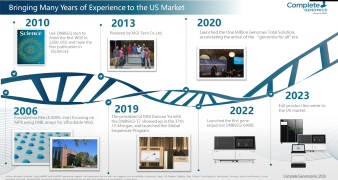
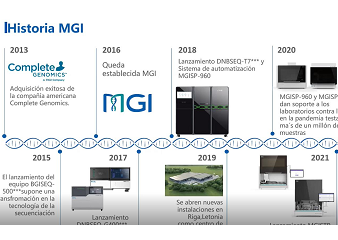
The current diagnosis of COVID-19 mainly depends on epidemiological history, clinical manifestations, laboratory examination, and imaging appearance. The clinical confirmation of suspected cases requires laboratory molecular tests on respiratory specimens or blood specimens - real-time fluorescent PCR or viral gene sequencing. The laboratory procedures of the two detection methods similarly include the nucleic acid extraction from the inactivated sample, the nucleic acid pretreatment based on different detection methods, the automatic detection, and the identification result generation based on software analysis. In the webinar, MGI will introduce a total package that includes one-stop nucleic acid extraction and high-throughput sequencing.

MGI Central & Eastern Europe District Manager and Automation Specialist
If you have any questions, you can send an email to:
MGI_marketing@mgi-tech.com



 Sequencer Products: SEQ ALL
Sequencer Products: SEQ ALL














 Technologies
Technologies Applications
Applications Online Resources
Online Resources Data Bulletins
Data Bulletins Service & Support
Service & Support Introduction
Introduction Newsroom
Newsroom Doing Business With Us
Doing Business With Us Creative Club
Creative Club