SHENZHEN, China, 28 April 2023 – MGI Tech Co. Ltd. (MGI), a company committed to building core tools and technology to lead life science, today shared that a total of nearly 60,000 samples have been sequenced among 21 institutes and over 10 participating nations throughout Europe, as part of the Million Microbiome of Humans Project (MMHP) that was officially launched in 2019.

The project was launched as a joint effort by the Karolinska Institute of Sweden, Shanghai National Clinical Research Center for Metabolic Diseases in China, the University of Copenhagen in Denmark, Technical University of Denmark, MetaGenoPolis at the National Research Institute for Agriculture, Food and Environment (INRAE) in France, and the Latvian Biomedical Research and Study Center. Relying on MGI's core DNBSEQ™ technology, MMHP aims to sequence and analyze microbial DNA from a million human samples to construct a microbiome map of the human body and build the world's largest human microbiome database.
"Countless studies have highlighted the importance of the microbiome in human health and disease. Yet, our knowledge of the composition of the microbiome in different parts of the body across countries, ages, sexes, and in relation to human health and disease remains limited," said Duncan Yu, President of MGI. "Through MMHP, we are pushing forward microbial metagenomic research while empowering researchers within the microbiology community with access to MGI's innovative sequencing technology. Despite a brief interruption by the COVID-19 pandemic, we are delighted to see such a monumental milestone merely four years into the project."
The rise of microbial metagenomic sequencing
Since the first description of human microbiome was published in 2010, the field of human microbiome has moved fast from sampling hundreds of individuals to thousands. Advances in genome sequencing has enabled researchers to better characterize the composition of the microbiome through identification of unculturable microbes. It has also allowed them the opportunity to study how the microbiome influences the development of some cancers and drug responses.
Metagenomics, coupled with high-throughput sequencing technologies, have revolutionized microbial ecology. Today, metagenomic sequencing has become both a powerful and popular tool for identifying and classifying complex microbial communities. It facilitates accelerated discovery of new markers that translate to virulence or antibiotic resistance, as well as de novo discovery and characterization of novel species and assembly of new genomes. Besides human microbiome, it is highly applicable in agricultural microbiome studies, environmental microbiome studies, pathogen surveillance and identification, and monitoring of antimicrobial resistance genes.
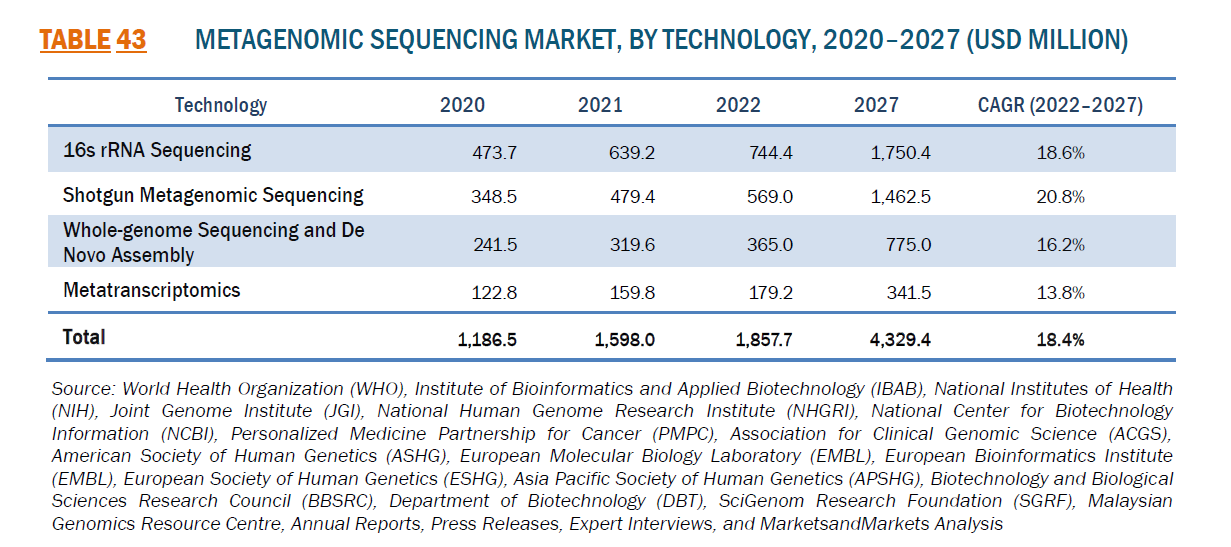
Indeed, the global metagenomic sequencing market was estimated to be worth USD 1.86 billion in revenue in 2022 and is poised to reach USD 4.33 billion by 2027, growing at a CAGR of 18.4% during the forecast period. In particular, Europe and Africa account for approximately 29.7% market share from the globe, ranking second after North America at 45.6%. Thanks to continuous technological innovations in high-throughput sequencing platforms, the metagenomic sequencing market within Europe and Africa is projected to grow from USD 551.7 million in 2022 to 1.29 billion by 2027, presenting huge market opportunities and providing local institutions with the impetus to invest and get involved.
An optimized workflow with MGI's cutting-edge technology
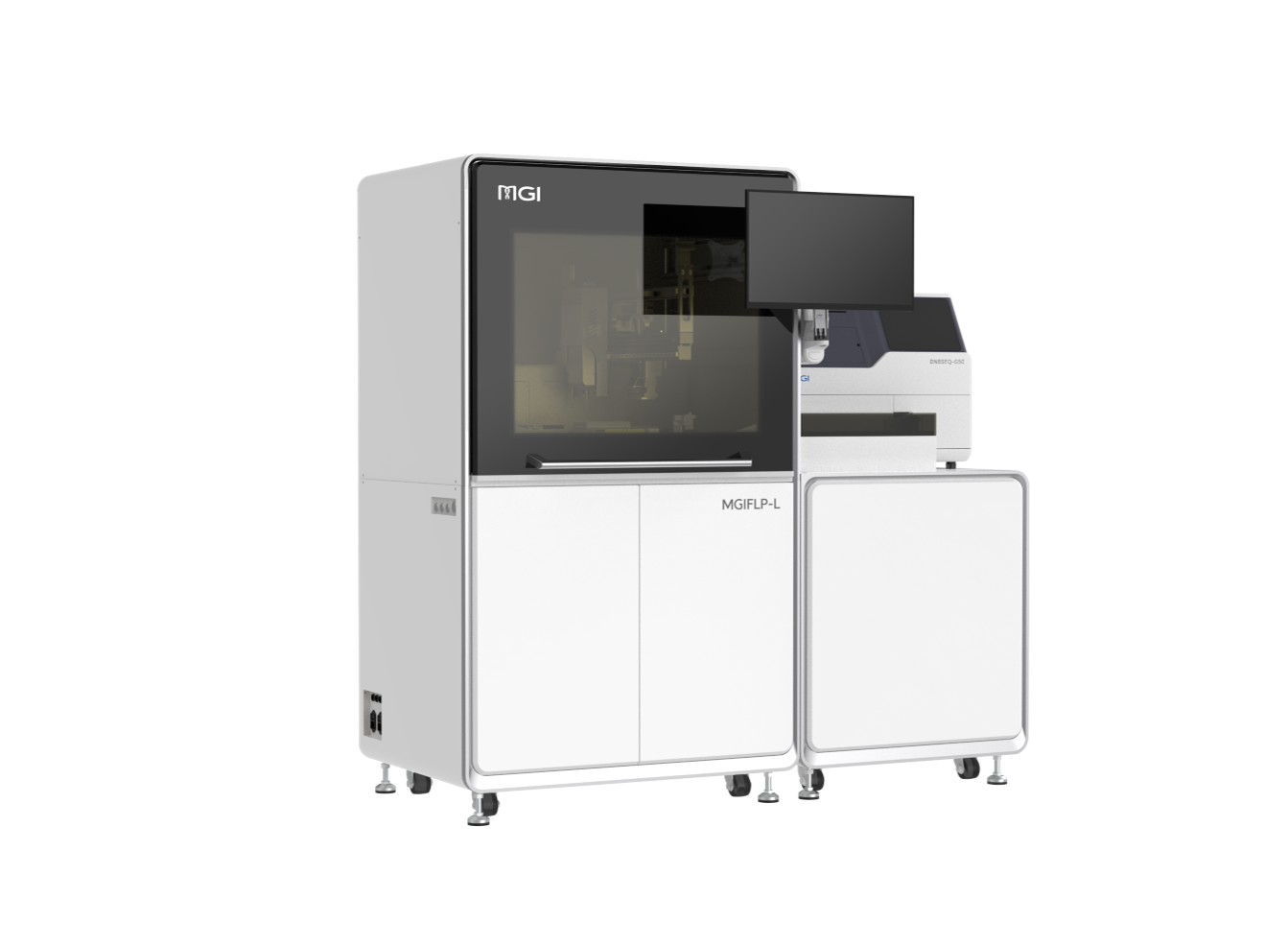
Equipped with MGI's innovative lab systems, the MMHP Consortium guarantees high-throughput processes, extreme precision, and high quality data output. The dedicated, one-stop workflow begins with sample transfer on MGISTP-7000* high-throughput automated sample transfer processing system. It then goes through nucleic acid extraction and library preparation on MGISP-960 high-throughput automated sample preparation system, a flexible and fully automated workstation capable of processing 96 samples per run. MGISP-960's fully automatic operation design allows DNA extraction of 50,000 samples per year and library preparation of 25,000 samples per year. MGISP-Smart 8, the professional automated pipetting robot, equipped with an independent 8 pipetting channel can be used for the pooling, normalization and DNB making. Lastly, DNBSEQ-T7* ultra-high throughput sequencer and DNBSEQ-G400* versatile benchtop sequencer enables an efficient, productive, and streamlined sequencing experience.
"We are very focused on data quality, cost and time. After contrasting DNBSEQ™ technology by MGI with other sequencing technologies, we are convinced that MGI’s products have met high industry standards and provide a very good user experience," commented Professor Lars Engstrand, Research Director of Center for Microbial Translational Research (CMTR) at Karolinska Institutet. "MGI's platforms have enabled our team to upgrade our original microbiological research from 16SrRNA gene amplicon sequencing to shotgun metagenomic sequencing. I look forward to introducing more equipment and super-large projects as human microbiome emerges as a crucial diagnostic and treatment method in precision medicine."
The next chapter in microbiomics
"Microbiomics will be part of precision medicine in the future, and data from the microbiome biobank that will result from MMHP will be leveraged for therapeutic R&D," said Professor Stanislav Dusko Ehrlich of University College London, UK. "With 21 public and private institutions and 10+ countries currently involved in MMHP, we are actively looking for more research groups to take part in this landmark international microbiological research partnership and help generate the world's biggest free-access human microbiome database."
Since the inception of MMHP, MGI has played an important role in providing the program with state-of-the-art research platforms and technologies. Now entering its second phase towards sequencing and analyzing a final total of one million samples, the project welcomes further exchange and participation from relevant organizations to jointly promote research and applications of cutting-edge translational medicine in the field of microbiome.
About MGI
MGI Tech Co. Ltd. (MGI), headquartered in Shenzhen, is committed to building core tools and technology to lead life science through intelligent innovation. Based on its proprietary technology, MGI focuses on research & development, production and sales of sequencing instruments, reagents, and related products to support life science research, agriculture, precision medicine and healthcare. MGI is a leading producer of clinical high-throughput gene sequencers*, and its multi-omics platforms include genetic sequencing*, medical imaging, and laboratory automation. MGI's mission is to develop and promote advanced life science tools for future healthcare. For more information, please visit the MGI website or connect with us on Twitter, LinkedIn or YouTube.
*Unless otherwise informed, StandardMPS and CoolMPS sequencing reagents, and sequencers for use with such reagents are not available in Germany, Spain, UK, Sweden, Italy, Czech Republic, Switzerland and Hong Kong (CoolMPS is available in Hong Kong).
*For Research Use Only. Not for use in diagnostic procedures (except as specifically noted).


 Sequencer Products: SEQ ALL
Sequencer Products: SEQ ALL










 Technologies
Technologies Applications
Applications Online Resources
Online Resources Data Bulletins
Data Bulletins Service & Support
Service & Support Introduction
Introduction Newsroom
Newsroom Doing Business With Us
Doing Business With Us Creative Club
Creative Club