As the clinical demand for tumor gene testing continues to grow, in-hospital testing has gradually become the mainstream for high-throughput sequencing applications. However, the complexity and stability of the sequencing library preparation process can pose challenges.
01
Manual operation affects the efficiency and stability of library construction
To perform the work of manual library construction requires a lab technician's full attention from 8 am to 6 pm.
02
Difficult laboratory construction
The conventional library construction setting requires at least four laboratories and has strict construction requirements, which most hospitals are unable to meet.
Solving the problem
MGI considered how to solve these problems and promote the implementation of high-throughput sequencing in hospitals. In 2017, MGI took action to develop a complete package for the industry (including oncology applications) and within two years produced a highly mature and fully validated automated library building system for tumor detection in hospital applications.
2017: MGISP-100 developed for hospitals was officially released
The automated library preparation equipment had to meet very specific requirements to be deployed in the hospital: the ability to process 8-16 samples at a time, a size small enough to be placed on the desktop, and manual operation completed within 10 minutes. MGI started from the needs of the hospital, and after two years of research and development and product iteration, MGISP-100 was released at the 12th International Conference on Genomics (ICG-12).
2018: Special R&D for oncology applications, and verification of tumor detection kits based on hybrid capture technology completed
MGISP-100 provides an automated library package for the most popular hybrid capture technology in the tumor field. The entire process of construction, hybrid capture, and DNB preparation is automated, minimizing manual time.
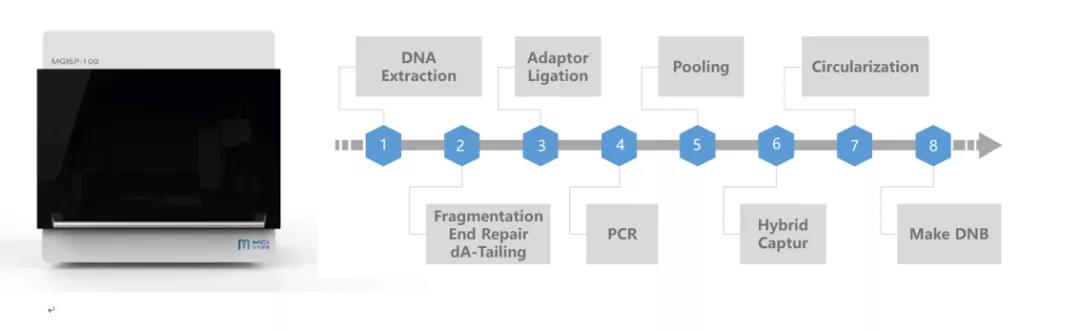
The MGISP-100-based "Hua Fei Ran Lung Cancer Detection Kit" supports 100ng of FFPE samples and can detect frequency mutations as low as 1%, and the success rate of library construction is above 99%.
2019: Expanded platform versatility, third-party multiple PCR targeting kits
Can MGISP-100 bring more surprises to the market? In 2019, MGI cooperated with third-party tumor detection kit manufacturers Paragon Genomics and Pillar Biosciences to develop tumor detection based on MGISP-100 multiple PCR targeting technology, expanding the range of applications.
Analysis process and performance demonstration using Paragon kit as an example:
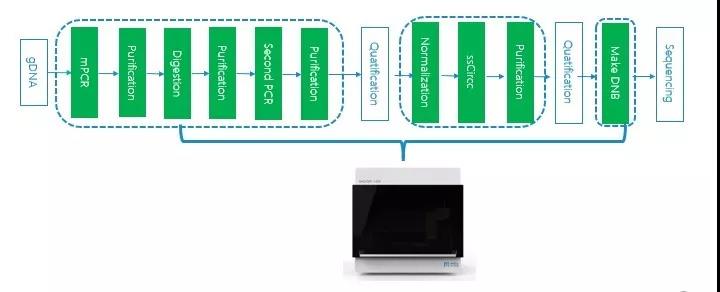
At present, MGISP-100 is a highly mature and fully validated automated library construction system that can be used for tumor detection, and has several important advantages:
1. Released for two years in the industry
2. Application development: comprehensive adaptation to hybrid capture and multiple PCR technologies
3. The first company in the world to obtain a registration certificate: February 2019 NMPA certification
4. Practical clinical application: introduced in 2019, it has been widely used in the hospital



2020: Validate more applications and expand third-party cooperation
In 2020, MGISP-100 will cooperate with more third-party tumor kit manufacturers to develop more sequencing tumor packages. It will develop adaptations, including other hybrid capture technologies, and expand the adaptability of sequencing platforms. We look forward to working with more customers to establish cooperation and jointly promote the implementation and application of tumor detection in hospitals.



 Sequencer Products: SEQ ALL
Sequencer Products: SEQ ALL















 Technologies
Technologies Applications
Applications Online Resources
Online Resources Data Bulletins
Data Bulletins Service & Support
Service & Support Global Programs
Global Programs Introduction
Introduction Newsroom
Newsroom Doing Business With Us
Doing Business With Us Creative Club
Creative Club